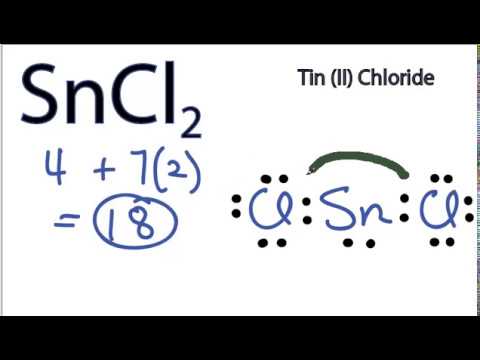
SOLVED: Under some conditions SnCl2 can simultaneously behave as both a Lewis acid and a Lewis base. Suggest a molecule that might be formed under these conditions. What is the hybridization on

Give an example of a compound that could be formed when SnCl2 acts as a Lewis acid. What is the - Brainly.in

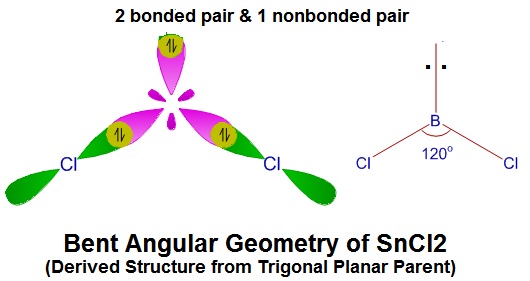
SOLVED: Which of the following species has/have the bent molecular geometry? How do you know? SnCl2, HCN, and H2S. To support your answer, hand draw the Lewis structure of each species with