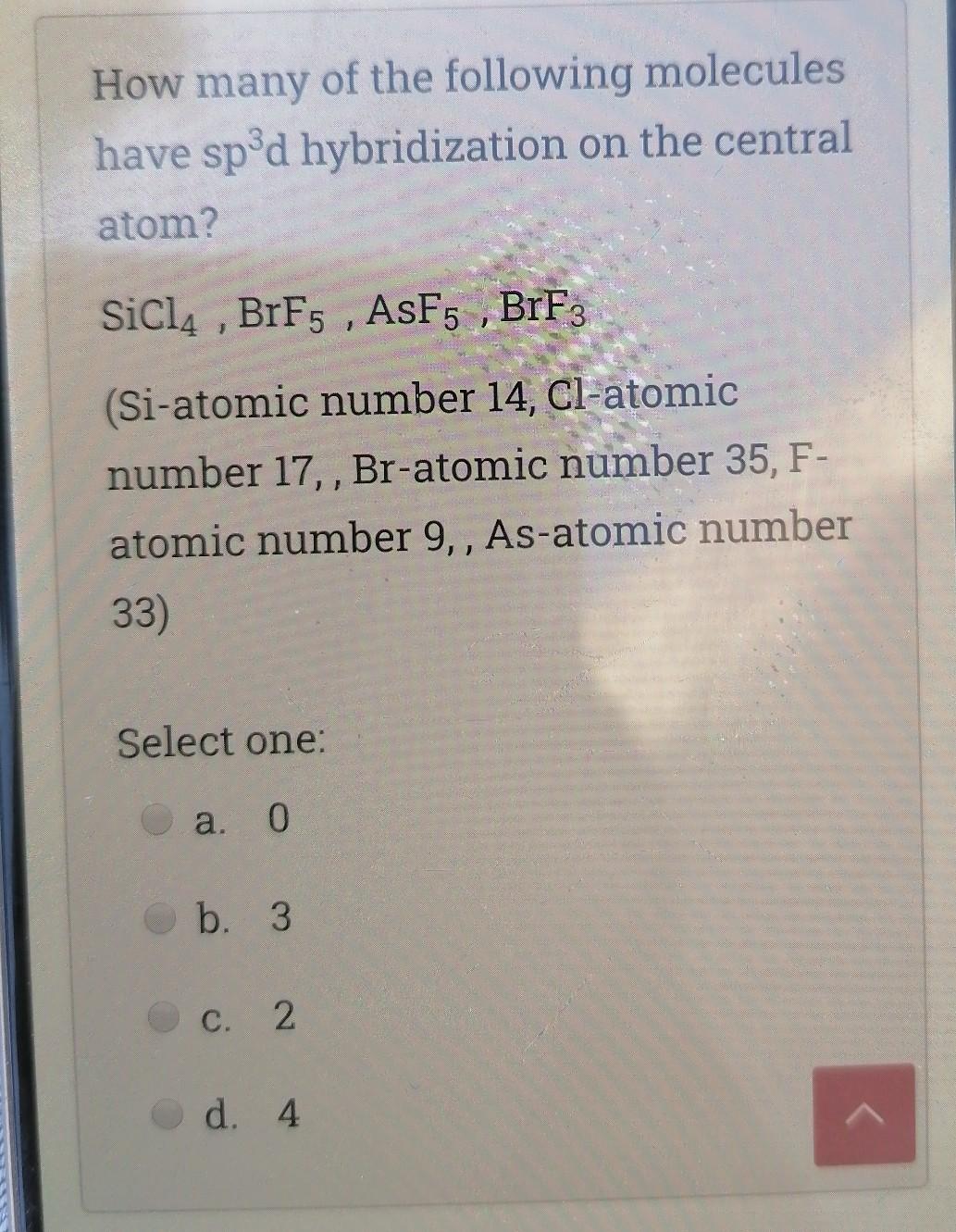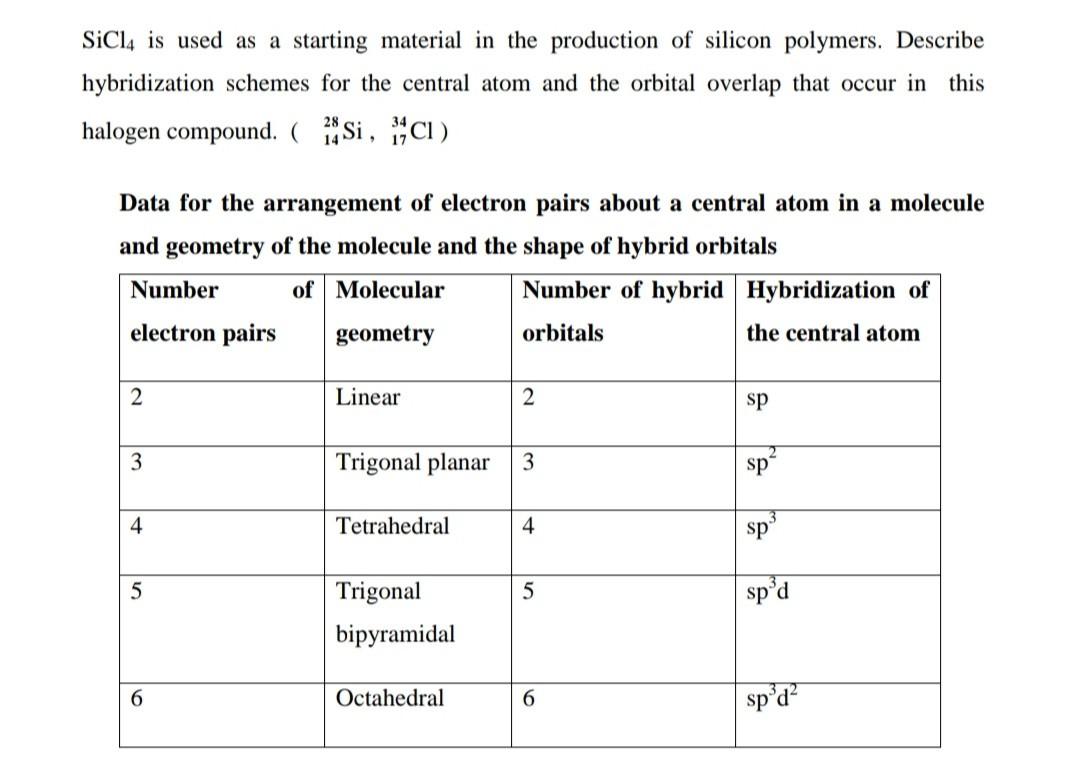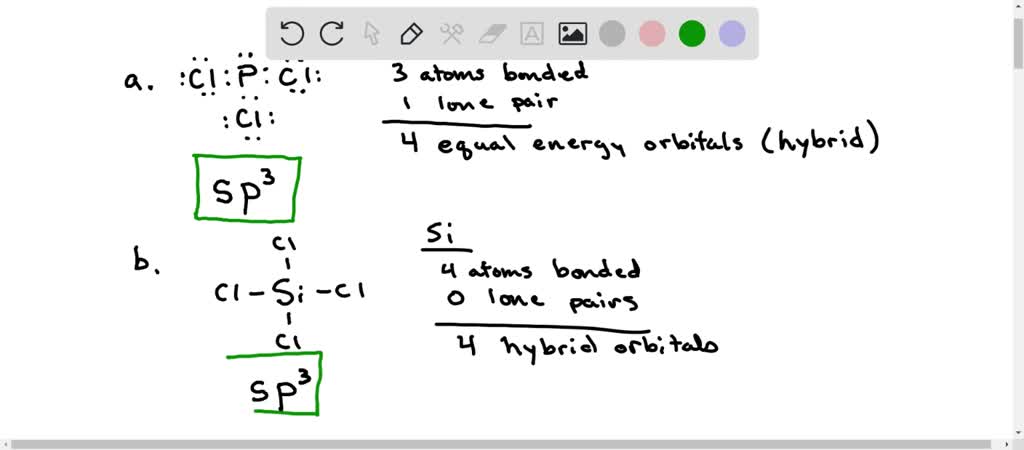
SOLVED: 10.50. What hybrid orbitals would be expected for the central atom in each of the following molecules or ions? a. PCl3 b. SiCl4 c. BeF2 d. SO2
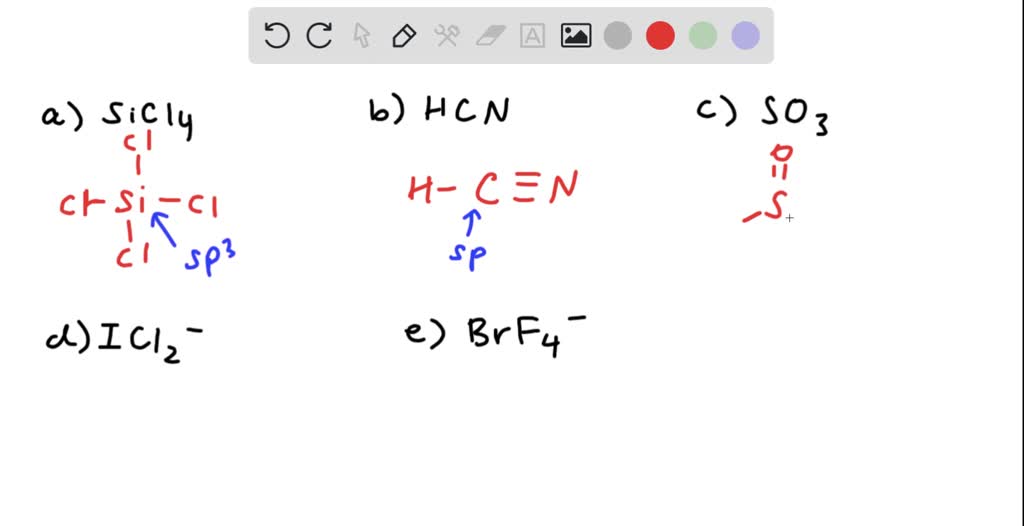
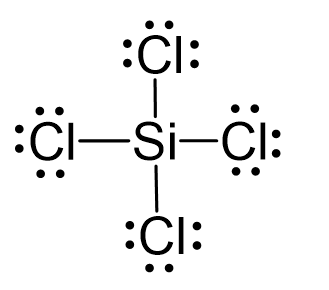
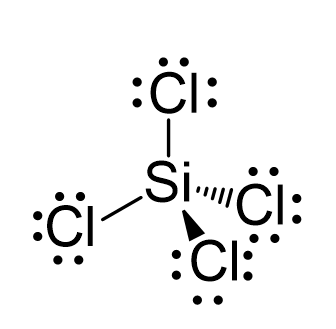
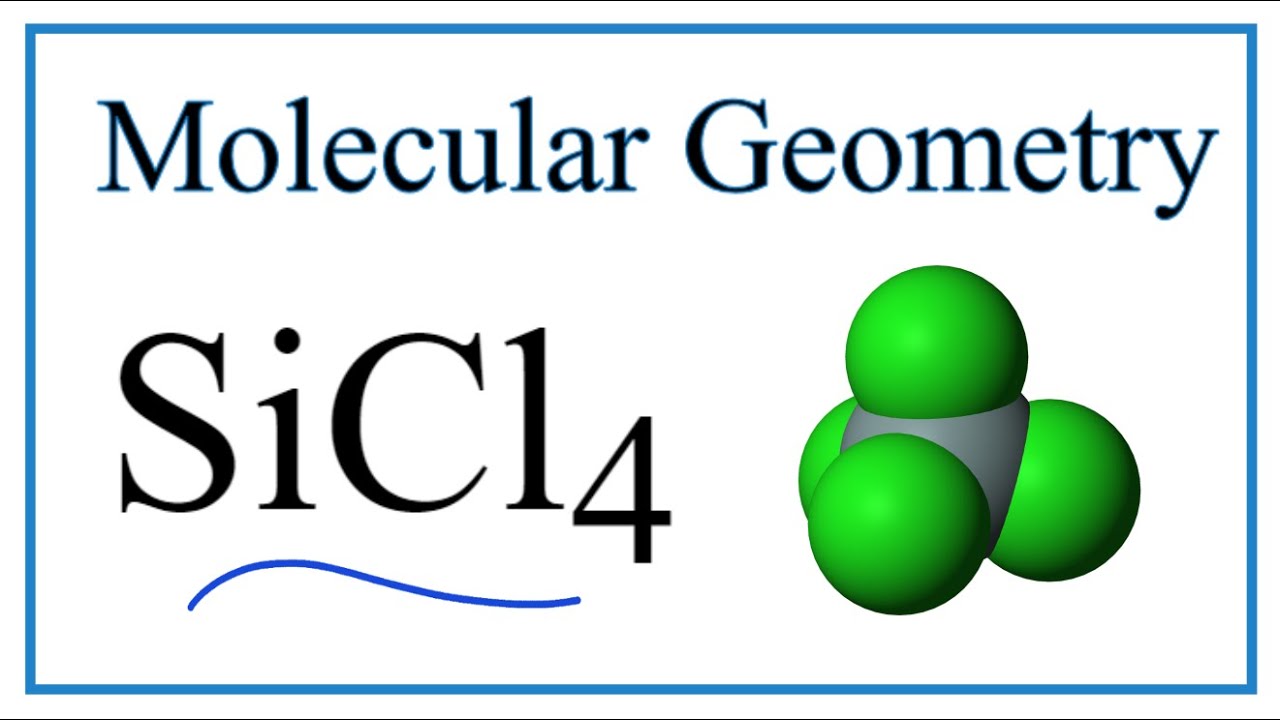
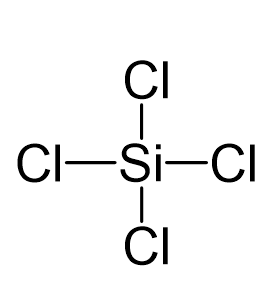
Silicon tetrachloride SiCl4: Molecular Geometry - Hybridization - Molecular Weight - Molecular Formula - Bond Pairs - Lone Pairs - Lewis structure –
We all know that CCl4 and SiCl4 both compound remains in Tetrahedral structure , with a hybridization of sp³ in the central atom. Now , Si is a much larger atom than