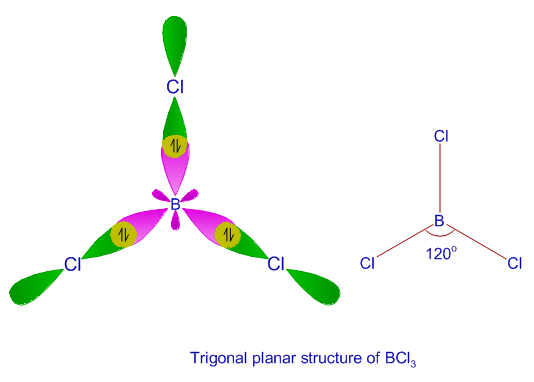
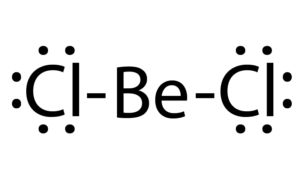what is Hybridisation? Based on Hybordisationwrite the formation of given below molecules?1) Becl2 2)NH3 3) - Brainly.in
47. What is the hybridization of BeCl2 in solid and vapour stage and give me the structures of both.
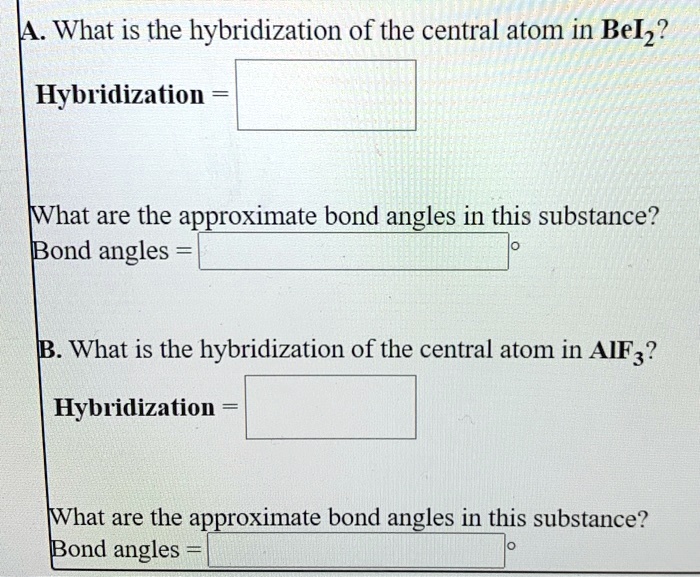
SOLVED: What is the hybridization of the central atom in BeCl2? Hybridization What are the approximate bond angles in this substance? Bond angles What is the hybridization of the central atom in
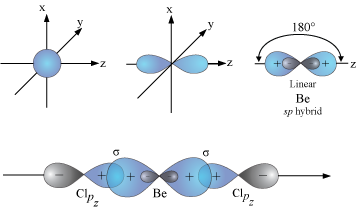
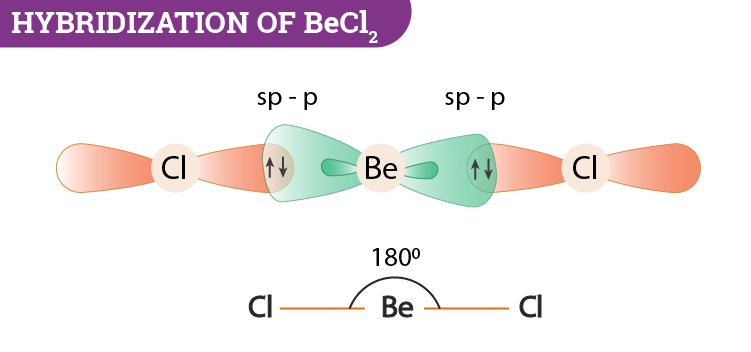
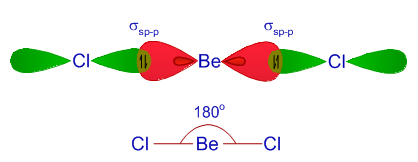
VSEPR theory tells that the valence electron pairs stay as far from each other as possible. For example, in BeCl_2 the two electron pairs of the two bonds are far apart so

BeCl2 Lewis structure, Molecular geometry, Hybridization, Bond angle and shape - Geometry of Molecules

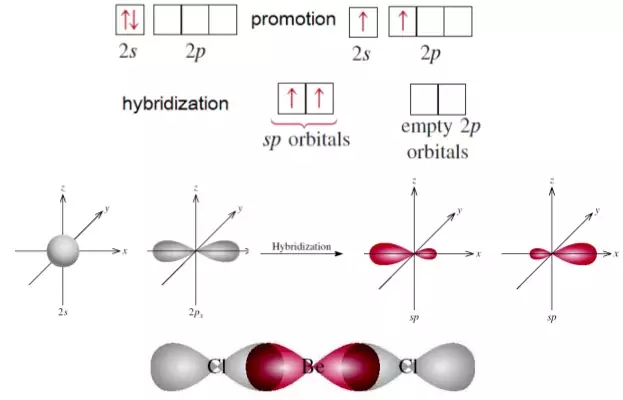
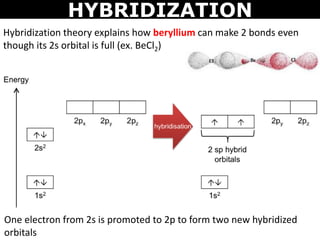
Hybridization state of beryllium atom in BeCl_2 molecule is x. The of hybridization changes to y when BeCl_2 transforms to the solid state. Then x,y are respectively:sp,sp^2sp,sp^3sp^2,sp^3sp,sp

Sir please explain this example of BeCl2 of sp hybridization - Chemistry - - 16269645 | Meritnation.com