![The hybridization of Fe in K4[Fe(CN)6] complex is | Coordination Master Series | Valence Bond Theory - YouTube The hybridization of Fe in K4[Fe(CN)6] complex is | Coordination Master Series | Valence Bond Theory - YouTube](https://i.ytimg.com/vi/E5Dr4k4sbD0/maxresdefault.jpg)
The hybridization of Fe in K4[Fe(CN)6] complex is | Coordination Master Series | Valence Bond Theory - YouTube
![The hybridization and geometry of [Fe(CO)_4]^{2-} are :sp^3d, TBPsp^3, tetrahedralsp^3, TBPdsp^2, square planar The hybridization and geometry of [Fe(CO)_4]^{2-} are :sp^3d, TBPsp^3, tetrahedralsp^3, TBPdsp^2, square planar](https://search-static.byjusweb.com/question-images/toppr_ext/questions/265623_249064_ans_855d6ece239c47e2a7778d0556da172e.png)
The hybridization and geometry of [Fe(CO)_4]^{2-} are :sp^3d, TBPsp^3, tetrahedralsp^3, TBPdsp^2, square planar

Valence bond theory of Coordination Compounds- Features, Hybridisation, Geometry, Examples, Limitation and FAQs of Valence bond theory.
![Welcome to Chem Zipper.com......: [Fe(CN)6]3- is weakly paramagnetic while [ Fe(CN)6]4- is diamagnetic, why ? Welcome to Chem Zipper.com......: [Fe(CN)6]3- is weakly paramagnetic while [ Fe(CN)6]4- is diamagnetic, why ?](https://blogger.googleusercontent.com/img/b/R29vZ2xl/AVvXsEisF7ewBdjRwV9P2QOqyrOrPdt_zrO-MMSNvO-Szjlwoi4icjioowahBpvcDJgYAQQl8Cf2_FbfZHE3MchyoCJfoOULYfJYhOc4iMtBwWEWgf5kpFqRCkoC3qm_8ZG4drbMZrTQdhjlxAM/s1600/1650694296671384-0.png)
Welcome to Chem Zipper.com......: [Fe(CN)6]3- is weakly paramagnetic while [ Fe(CN)6]4- is diamagnetic, why ?
![a) For the complex { left[ Fe{ left( CN right) }_{ 6 } right] }^{ 3- }, write the hybridization type, magnetic character and spin nature of the complex. (Atomic number : a) For the complex { left[ Fe{ left( CN right) }_{ 6 } right] }^{ 3- }, write the hybridization type, magnetic character and spin nature of the complex. (Atomic number :](https://search-static.byjusweb.com/question-images/toppr_ext/questions/994279_471862_ans_274df12034c14a6ea02085d9c811233c.bmp)
a) For the complex { left[ Fe{ left( CN right) }_{ 6 } right] }^{ 3- }, write the hybridization type, magnetic character and spin nature of the complex. (Atomic number :
![a For the complex [ F e CN 6]4 , write the hybridisation, magnetic character and spin type of the complex. At. number: Fe =26 b Draw one of the geometrical isomers a For the complex [ F e CN 6]4 , write the hybridisation, magnetic character and spin type of the complex. At. number: Fe =26 b Draw one of the geometrical isomers](https://byjus-answer-creation.s3.amazonaws.com/uploads/60e7df4424737982aa9fb83f_img_upload_solution_1652216200.png)
a For the complex [ F e CN 6]4 , write the hybridisation, magnetic character and spin type of the complex. At. number: Fe =26 b Draw one of the geometrical isomers
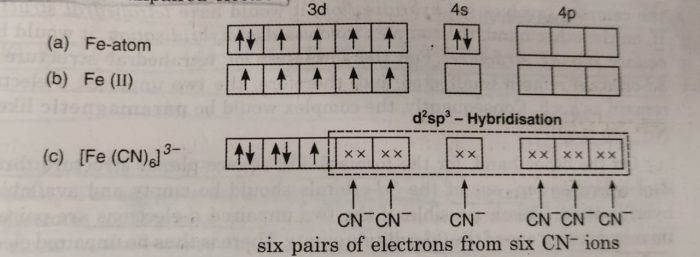
Valence Bond Theory For Bonding In Coordination Compounds - Chemistry, Class 12, Coordination Compounds
![For the complex [Fe(CN)6]3–, write the hybridization type, magnetic character and spin nature of the complex. - Chemistry | Shaalaa.com For the complex [Fe(CN)6]3–, write the hybridization type, magnetic character and spin nature of the complex. - Chemistry | Shaalaa.com](https://www.shaalaa.com/images/_4:cd81081959db46f9b02647463e8defe4.png)
![21. The EAN of Fe in [Fe(CN)6]3 is (1) 26 (3) 38 22. The hybridization o.. 21. The EAN of Fe in [Fe(CN)6]3 is (1) 26 (3) 38 22. The hybridization o..](https://storage.googleapis.com/filo-classroom-notes/thumb_classroom_28163530_F57BH.jpeg)
![For the complex ion of [Fe(CN)6]^(3-) : Show the hybridization diagr For the complex ion of [Fe(CN)6]^(3-) : Show the hybridization diagr](https://d10lpgp6xz60nq.cloudfront.net/physics_images/GRU_ISC_10Y_SP_XII_CHE_11_E01_039_S01.png)
![How would you account for the magnetic behaviour of [Fe(CN)(6)]^(3-) a How would you account for the magnetic behaviour of [Fe(CN)(6)]^(3-) a](https://d10lpgp6xz60nq.cloudfront.net/physics_images/AAK_T5_CHE_C19_SLV_008_S02.png)
![The hybridization of `Fe` in `K_4[Fe(CN)_6]` complex is: - YouTube The hybridization of `Fe` in `K_4[Fe(CN)_6]` complex is: - YouTube](https://i.ytimg.com/vi/2E532r8xHTg/maxresdefault.jpg)
![Kannada] With the help of VBT explain the geometry of K4 [Fe(CN)6 and Kannada] With the help of VBT explain the geometry of K4 [Fe(CN)6 and](https://d10lpgp6xz60nq.cloudfront.net/physics_images/OSW_SP_CHE_XII_C09_E02_032_S01.png)
![Fe(CN)6]4– is diamagnetic while [FeF6]4– i Fe(CN)6]4– is diamagnetic while [FeF6]4– i](https://www.zigya.com/application/uploads/images/chen12070386_571486ba519b3.png)
![The hybridization and magnetic nature of [MnCN6]4 and[FeCN6]3 , respectively are: The hybridization and magnetic nature of [MnCN6]4 and[FeCN6]3 , respectively are:](https://byjus-answer-creation.s3.amazonaws.com/uploads/7692Chemistry_62bc3161cac8b02f5af0a2d2_c.jpg_img_upload_solution_2022-09-23%2017:22:54.161282.png)

![Fe(CN)6]4– is diamagnetic while [FeF6]4– i Fe(CN)6]4– is diamagnetic while [FeF6]4– i](https://www.zigya.com/application/uploads/images/chen12070390_5714b9d19ba87.png)
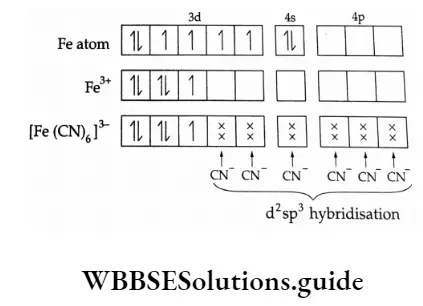
![Solved [Fe(H2O)6]2+,[Fe(CN)6]4− coordination compounds shown | Chegg.com Solved [Fe(H2O)6]2+,[Fe(CN)6]4− coordination compounds shown | Chegg.com](https://media.cheggcdn.com/media/c50/c50287c8-e5c4-44a6-a107-38c183c38ba4/phpPgEih1)
![Writen the hybridization, shape and magnetic character of [Fe(CN)(6)]^ Writen the hybridization, shape and magnetic character of [Fe(CN)(6)]^](https://d10lpgp6xz60nq.cloudfront.net/physics_images/SB_CHM_XII_OD_I_2016_E01_019_S01.png)